Precision medicine is an approach that takes into account the inflence of individuals’ genes, environment, and lifestyle exposures to tailor interventions. Here, we describe the development of a robust precision cancer care platform that integrates whole-exome sequencing with a living biobank that enables high-throughput drug screens on patient-derived tumor organoids. To date, 56 tumor-derived organoid cultures and 19 patient-derived xenograft (PDX) models have been established from the 769 patients enrolled in an Institutional Review Board–approved linical trial. Because genomics alone was insuffiient to identify therapeutic options for the majority of patients with advanced disease, we used high-throughput drug screening to discover effective treatment strategies. Analysis of tumor-derived cells from four cases, two uterine malignancies and two colon cancers, identifid effective drugs and drug combinations that were subsequently validated using 3-D cultures and PDX models.
WES Is Insuffiient to Identify Clinically Targetable Alterations for Many Advanced Cancer Types
Our institute established the EXaCT-1 Test, a WES-based precision medicine platform designed to inform therapeutic decision-making for patients with cancer. To date, the EIPM has sequenced and analyzed 769 tumor–normal pairs from an array of different primary and metastatic tumor sites from 501 patients, the majority of whom had advanced disease (Fig. 1A).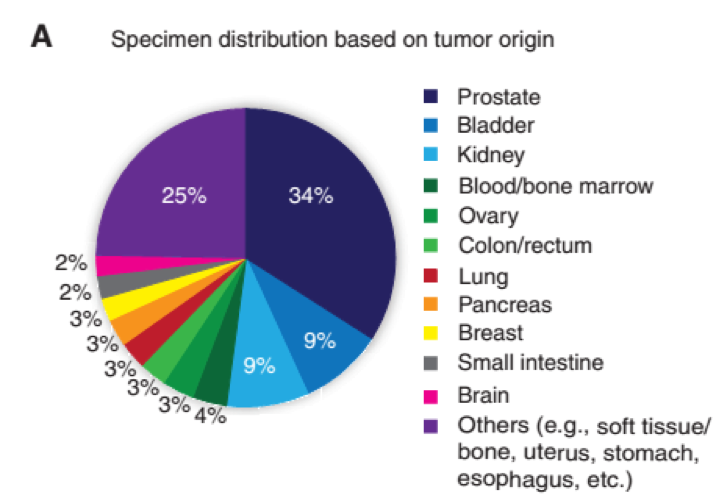
WES identifid alterations involving known cancer genes in 95.8% (737/769) of the analyzed specimens. In our cohort, there were FDA-approved drugs identifid for 0.4% (3/737) of patients. Based on an expanded list of targeted therapies available at My Cancer Genome (30), 9.6% (71/737) of the analyzed patients had potentially targetable cancer gene alterations, though without current FDA-approved drug indication (Fig. 1B and C).
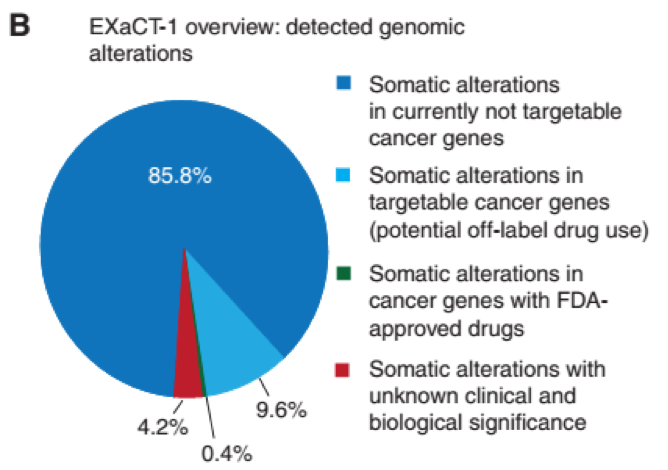

The most frequently mutated cancer genes with singlenucleotide variants (SNV)/indels in our cohort (≥5%) were TP53 (37.4%), APC (11.3%), NOTCH1 (8.4%), EGFR (6.7%). The most common somatic copy-number aberrations (SCNA; ≥18%) were seen in CDKN2A (25.3%), RB1 (24.9%), WRN (24.3%), PCM1 (22.4%).
Together, these data suggest that WES—although highly informative for some cancers with targetable mutations—is insuffiient to nominate therapeutic alternatives in many advanced cancer types.
Patient-Derived Tumor Organoids and Xenografts as Tools for Precision Cancer Care
To complement the genomic information and to provide therapeutic options for patients, we integrated personalized PDTO drug screens and PDX generation into our platform (Fig. 2).
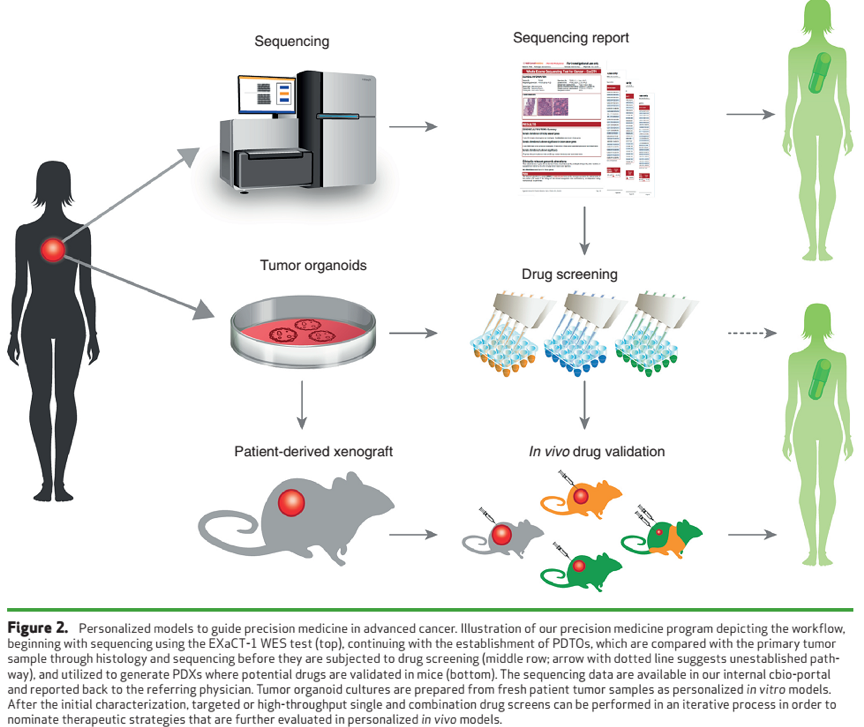
This platform thereby promotes the discovery of novel therapeutic approaches that can be assessed in clinical trials and provides personalized therapeutic options for individual patients where standard clinical options have been exhausted.
信息来源:
Pauli C, Hopkins BD, Prandi D, et al. Cancer Discov 2017,7(5):462-477